The interest of our laboratory concerns the cellular and molecular mechanisms that underlie neuronal maintenance and plasticity throughout life. Complex intracellular logistics are required to form and maintain neurons. Their processes are elaborate structures that cover vast territories, allowing neurons to create thousands of connections in a highly ordered manner, key to brain function. We aim to understand how neurons integrate the different extracellular cues that they receive at distinct parts of the cell in time and space to maintain and adapt their structure and connectivity in a tightly regulated manner. To this end, we study the relationship of short- and long-range trafficking and signalling, and how these events ultimately converge to initiate changes in neuronal morphology and physiology in health, tauopathy and neurodiversity. To address these questions, we work with rodent primary neuronal cultures. We employ a combination of high-resolution live and fixed cell optical microscopy as well as biochemical and molecular biological approaches. Currently active research projects are:
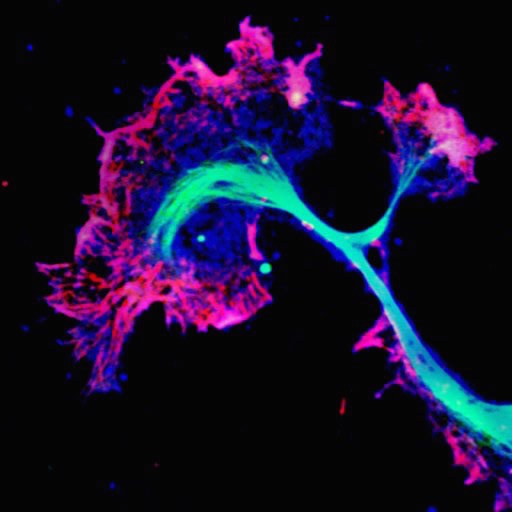
Signalling across cellular distances in neurodegeneration. Much of the initial dysfunction in neurodegeneration occurs within or adjacent to distal synaptic sites, a long distance from the soma, which is the site of gene expression regulation. This project investigates how retrograde injury signals, initiated by a disintegrating axonal structure, provoke somatic responses, and what the downstream consequences are. We are currently assessing how identified gene expression responses interact with the developing tau pathology. We further try to understand the underlying mechanisms by which disease-linked tau mutations impact local and long-range neurotrophic signalling, which is key to healthy neuronal plasticity and impaired early in dementias.
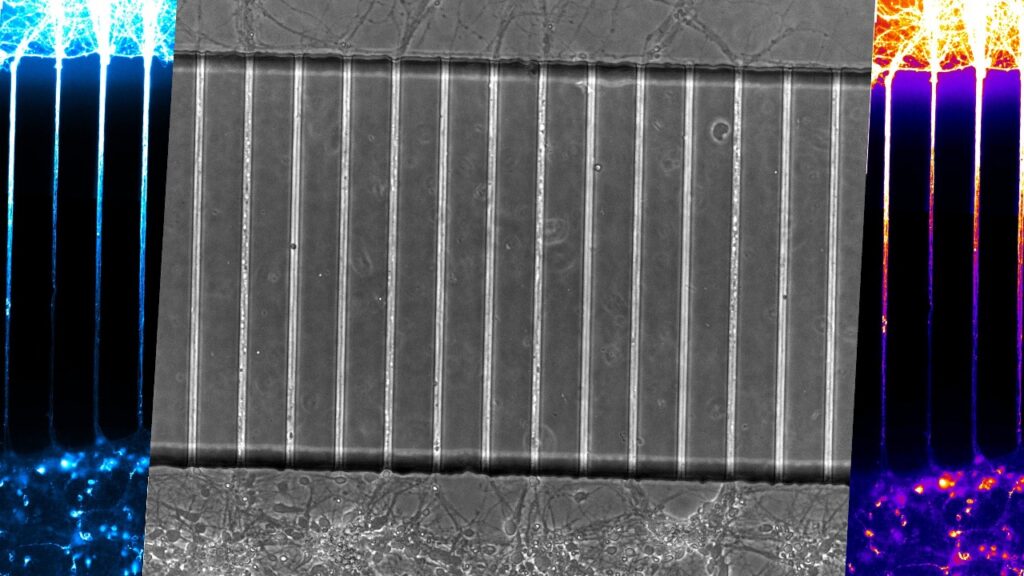
Investigating cellular mechanisms that underlie tau propagation across neurons. The aim of this project is to understand the cellular mechanisms by which pathology spreads across the brain, particularly pathogenic tau. We developed a new culture platform and demonstrated that neurons remain viable for prolonged periods of time in presence of pathogenic tau. More recently we used protein engineering to generate probes that now allow us to resolve the individual steps in tau transmission across neurons.
Post-translational modifications that control neuronal network activity. This project is a collaboration with Dr Yihua Wang at the University of Southampton, an expert in lung fibrosis and stable post-translational modifications. Using calcium imaging we found that hydroxylation alters neuronal network activity and are currently investigating the molecular targets that are hydroxylated to understand the underlying mechanisms. Interestingly, many of the candidates that harbour a consensus sequence for hydroxylation are implicated in neuropsychiatric disorders.
Molecular mechanisms underlying autism spectrum conditions. The aim of this project is to understand molecular determinants of neurodiversity. In collaboration with Dr James Dillon and Prof. Vincent O’Connor at the University of Southampton, we use C.elegans as a platform to screen high risk genes selected from the SFARI database for impact on sensory integration using a range of behavioural assays. Mutants that show a behavioural phenotype independent of locomotion deficits will be taken into mammalian neuronal cultures to unpick the underlying molecular mechanisms, including potential effects on synaptic vesicle cycling and synchronised network activity.